Water Electrolysis for Hydrogen, Water Treatment, and Carbon Sequestration.
john@h2o-c.com
(814) 574-2649
(814) 574-2649
Water electrolysis produces two different types of products at the two different electrodes of an electrolytic cell, electro-reduced water (ERW) and reactive oxygen species (ROS). ERW and ROS have application in 1) the production of hydrogen as an energy carrier for clean energy, 2) water treatment, and 3) carbon sequestration.
ERW and ROS are produced by electrolysis of water or brine in an electrolytic cell. Electro-reduced water (ERW) is produced at the cathode subjected to negative voltage, and its co-component, reactive oxygen species (ROS), is produced at the anode subjected to positive voltage. An electric current is supplied to an anode half-cell where electrons are removed and oxidation occurs, creating the reactive oxygen species (ROS), including oxygen, chlor-oxides, and chlor-acids. The electrons flow to a cathode half-cell where reduction occurs, creating ERW components, including hydrogen and hydroxide.
The electrolysis can be performed on both freshwater and brines. Both produce hydrogen and oxygen. However, brine electrolysis — usually referred to as chlor-alkali electrolysis because it most commonly involves naturally occurring alkali chloride salts (e.g. NaCl) — produces other byproducts. These byproducts can be useful, depending upon the circumstances and goals of the environmental projects. Part of the mission of Carbon Negative Water and Energy is to assess the water quality for these applications, then arrange by referral the technology for the project’s specific needs. Please review the information provided below for examples of specific applications:
Freshwater electrolysis
Chlor-alkali electrolysis
Hydrogen production for clean energy
Health
FAQs

Freshwater Electrolysis
Hydrogen production for clean energy
Electrolysis is used to make industrial levels of hydrogen for fuel cells. The most efficient electrolysis of hydrogen requires de-ionized (pure) water to make a KOH (potassium hydroxide) solution. The electrolysis process extracts hydrogen gas ( H2 ) and oxygen gas ( O2 ) from the water, and the KOH is recycled.
Anode oxidation: 2H2O + 4KOH ==> O2 + 4H+ + 4OH– + 4K+ + 4e– ==> O2 + 4H2O + 4K+ + 4e–
( note that acid, H+produced from the oxidation, reacts with hydroxide anion, OH– dissociated from KOH, to reform water)
( note that acid, H+produced from the oxidation, reacts with hydroxide anion, OH– dissociated from KOH, to reform water)
Cathode reduction: 4H2O + 4K+ + 4e– ==> 2H2 + 4OH– + 4K+ ==>2H2 + 4KOH
( note that hydroxide, OH– produced from the reduction, re-associates with the potassium cation, K+, to recycle KOH )
( note that hydroxide, OH– produced from the reduction, re-associates with the potassium cation, K+, to recycle KOH )
Hydrogen is an energy carrier that can be used to store renewable (continuously sourced) energies, such as solar or wind, for remote use, such as use in a hydrogen fuel cell electric car, or for use in other fuel cell applications when the renewable (continuously sourced) energy is unavailable. The primary energy source can thus be used continuously–rather than just continually–to power equipment on-site for other applications.

Water treatment
The electrolysis described above can be used to produce ERW and ROS for in-situ water treatment. Oxygen gas and other ROS agents such as ozone are oxidizing agents (electron acceptors) that can enhance aerobic respiration and breakdown of petroleum hydrocarbons and many other types of hydrocarbon contaminants. Hydrogen gas is a reducing agent (electron donor) that can enhance the breakdown of contaminants that require reduction, including very prevalent groundwater contaminants such as trichloroethene and perchlorate.
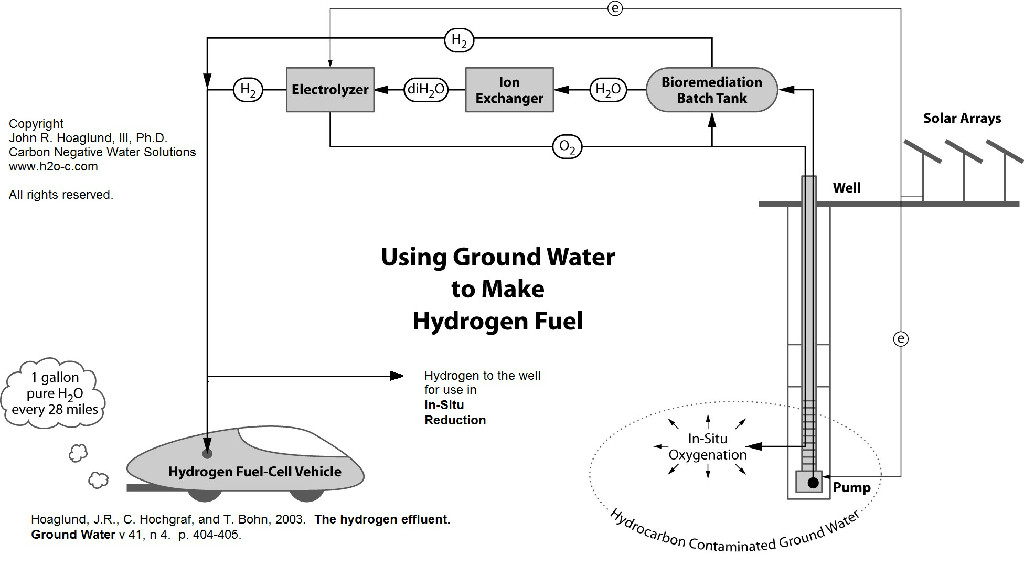
The use of hydrogen in in-situ remediation was the subject of the recent ESTCP Project ER-201027 summarized with the January, 2013 report Enhanced Attenuation of Unsaturated Chlorinated Solvent Source Zones Using Direct Hydrogen Delivery. As is common with water treatment generally, one of the key requirements to maximize the effectiveness of this remediation technique is to maintain pH. Using existing salts naturally occurring in the groundwater, electrolysis can create the acids and bases needed for pH management, in addition to the oxygen and hydrogen coming from the water itself. Thus unlike chemical mixing methods, electrolysis can produce these products from existing natural water chemistry without changing the natural salt content. Saline water electrolysis is described in the “carbon sequestration” topic below.
When combined with renewable (continuously sourced) energy and stored hydrogen, these treatment systems can be deployed in remote areas “off the grid” or other areas where providing electrical hookup is impractical.

Chlor-Alkali Electrolysis
Carbon Sequestration
ERW can provide a substrate for carbon sequestration, pulling CO2 into solution which can then either be taken up by biota (algae, plants, etc.) for biofuel, or reacted to form a solid mineral salt for more permanent sequestration.
The actual substrate for carbon sequestration is hydroxide alkalinity (a base). The following “kid scientists” experiment on the David Letterman Show vividly shows the reaction of CO2 with base to form bicarbonate / carbonate, locking up CO2 in solution. An explanation is provided in a caption:
The column starts as a basic solution; for simplicity assume it is sodium hydroxide, NaOH. The pH of the solution is in excess of 11 as indicated by the rich purple color of the pH indicator. A basic solution can be created by electrolysis using ERW technology (see below) from natural water salt chemistry; however it can also be added to solution from industry sources. Maddy Whirledge adds dry ice, which is carbon dioxide, to the solution, raising the “partial pressure of CO2“, Pco2. The pH changes into the neutral range, or even acidic range, pH 7 or less, as indicated by the yellow color of the pH indicator. Dave asks, “Why are we doing this?” Maddy replies, “Because it’s colorful.” LOL. Perhaps the most common answer to Dave’s question is that the experiment demonstrates a reaction that is part of “blood pH homeostasis.” By slowing breathing and allowing an increase of CO2 in the blood, the body can neutralize excess base into bicarbonate to strictly maintain its pH at 7.35. Perhaps the less well known answer to Dave’s question is that the experiment demonstrates a reaction that can be used to sequester CO2
The CO2 reacts to form bicarbonate and carbonate as follows:
bicarbonate (HCO3–)
1) H2O + NaOH + CO2 ==> Na+ + OH– + H+ + HCO3– ==> H2O + NaHCO3
Note: the right-most water is the neutralization of carbonic acid and base. A solid precipitate, baking soda, NaHCO3, may form.
1) H2O + NaOH + CO2 ==> Na+ + OH– + H+ + HCO3– ==> H2O + NaHCO3
Note: the right-most water is the neutralization of carbonic acid and base. A solid precipitate, baking soda, NaHCO3, may form.
carbonate (CO32-)
2) NaHCO3 + NaOH ==> 2Na+ + OH– + H+ + CO3– ==> H2O + 2Na+ + CO32-
Note: the right-most water is the further neutralization of carbonic acid and base. A solid precipitate, natron, Na2CO3 may form.
2) NaHCO3 + NaOH ==> 2Na+ + OH– + H+ + CO3– ==> H2O + 2Na+ + CO32-
Note: the right-most water is the further neutralization of carbonic acid and base. A solid precipitate, natron, Na2CO3 may form.
Though the pH changes as base is consumed, the TOTAL ALKALINITY DOES NOT CHANGE with the high Pco2, rather it shifts from a base component, NaOH, to the buffer components, HCO3– and CO32-. The result is a neutral solution of high alkalinity.
ERW can produce the base from saltwater. A salt is an ionic compound formed from the neutralization of an acid with a base. Various salts exist in natural waters. When electrolysis is applied to a salt water solution, the acid and base are recovered. Hydrogen in water at the anode can be liberated as acid, H+, up to an amount limited by available salt anions (chloride, etc.) required to balance the charge. Hydrogen and oxygen in water at the cathode can be liberated as base, OH– (hydroxide), up to an amount limited by available salt cations (sodium, potassium, calcium, etc.) required to balance the charge. As the amount of base increases at the cathode, it changes dissolved carbon dioxide into bicarbonate and carbonate that can form solids.
In general, the overall electrolysis reaction of water with a salt that creates base can be summarized as:
5H2O + 2NaCl ==> O2 + [Cl2 + H2O] | + 2NaOH + 3H2
The vertical bar on the right side of the equation separates the oxidized components of the anode half-cell on the left from the reduced components of the cathode half-cell on the right, including the base (NaOH in the example).
The anode half-cell can produce chlor-acids and/or chlor-oxides instead of the chlorine and water in brackets. These products are useful in water treatment.
5H2O + 2NaCl ==> O2 + HClOx + HCl | + 2NaOH + 3H2 where the “x” on the chlorine oxyanion can be 1 to 4.
Unlike chemical mixing methods, electrolysis produces these products from existing natural water chemistries without changing the natural salt content. Instead, the existing ions are prepared to combine with carbon and are then consumed, removing salts from the water. Hydroxide (OH–) is a component of alkalinity. Adding (or producing) more of the hydroxide component of alkalinity (raising the pH) moves dissolved carbon dioxide into bicarbonate and carbonate alkalinity components, as shown in this video for a water with a constant amount of dissolved CO2
Summarized by the graph below: If water is open to a constant CO2 gas pressure such as that from the atmosphere, adding base (raising the pH) increases the total alkalinity (blue line), which increases the amount of total carbon (red line) that can be dissolved into the water.
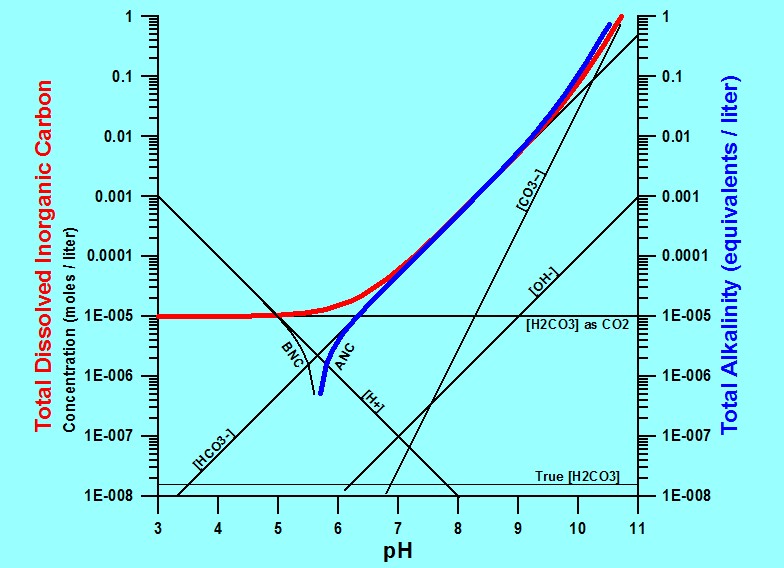
When total alkalinity and total dissolved inorganic carbon are high enough, carbonate minerals quickly form and precipitate as a very stable (permanent) carbon solids.
The H2CO3 as dissolved CO2, shown as a horizontal line on the graph, corresponds to the constant pressure of CO2 in the atmosphere. Significantly greater amounts of carbon can be sequestered if the water is subjected to higher pressures of CO2.
This is how the Earth removed most of the CO2 in its original atmosphere, and continues to do so as part of the multi-million year carbon cycle. We can mimic it.
Hydrogen Production for Clean Energy
As mentioned above, hydrogen is an energy carrier that can be used to store renewable (continuously sourced) energies, such as solar or wind, for remote use, such as use in a hydrogen fuel cell electric car, or for use in other fuel cell applications when the renewable (continuously sourced) energy is unavailable. The primary energy source can thus be used continuously–rather than just continually–to power equipment on-site for other applications.
With chlor-alkali electrolysis, different proportions of ROS species can be engineered, ranging from pure native chlorine (Cl2) and oxygen (O2) to various (x) chlorine oxides, ClOx, and if reacted with the hydrogen (H2), different proportions of hydrochloric acid (HCl) or hypochlorous acid (HOCl). HCl applied to native metals produces that metal’s chloride plus hydrogen gas, thus the hydrogen can be recovered and the chlorine neutralized to relatively innocuous chloride salt.
Chlor-alkali electrolysis produces different chlorine compounds depending upon desired proportions of ROS species, ranging from pure native chlorine (Cl2) and oxygen (O2) to various (x) chlorine oxides, ClOx, and if reacted with the hydrogen (H2), different proportions of hydrochloric acid (HCl) or hypochlorous acid (HOCl). Hydrochloric acid (HCl) has many uses across industry. HCl applied to native metals produces that metal’s chloride salt plus hydrogen gas, thus the hydrogen can be recovered and the chlorine neutralized to relatively innocuous chloride salt. Native chlorine (Cl2), the various (x) oxides of chlorine (ClOx), and hypochlorous acid (HOCl) are all used as disinfectants, including use for water sanitation, most notably bleach (NaOCl). Ammonium perchlorate (NH4ClO4) is used as solid rocket fuel, including historically in the Space Shuttle’s two main rocket boosters.

Dr. Hoaglund spends, and has spent, much of his career concerned with things we don’t want in our drinking water, cleaning up legally-adversarial contaminated messes. Searching for more life-affirming work, he wanted to turn at least some of his focus onto the healthy constituents that exist in natural waters, constituents that we DO want in our water, particularly the “minerals” (ions), alkalinity, and hydrogen that are known to be healthy in drinking water.
He found a growing number of scientific studies that are confirming that hydrogen and total alkalinity, two agents that are enhanced within electro-reduced water (ERW), have therapeutic effects for a number of diseases. Known causative mechanisms exist that can explain these therapeutic observations.
Hydrogen is an electron donor that dissolved in drinking water can act as an “anti-oxidant” to neutralize free radicals. A summary of free radicals and the physiological effects of hydrogen water consumption can be found at: http://www.molecularhydrogeninstitute.com/
Total alkalinity, also known as acid neutralizing capacity (ANC), consumed in drinking water, acts to neutralize acid within the body. The exact localization of the acid neutralization in the body is an area of active research and debate: 1) whether the acid neutralization can occur in the upper GI, lower GI, or other tissues by way of the bloodstream; and 2) whether the effects of the alkalinity are delivered directly, or by a sequence of physiological responses. The therapeutic effect of any acid neutralization on any of these tissues–particularly on any cancerous tissues–is also an area of active research and debate.
Both hydrogen and total alkalinity can alternatively be added to water by chemical mixing methods, rather than the electrolysis that produces ERW. However electrolysis can produce these therapeutic agents from existing natural water chemistry without changing the natural salt content that occurs when using chemical additives.
FAQs
1) What is alkalinity? What does “alkaline” mean?
2) What is total alkalinity (ANC)?
3) Does alkalinity change with temperature, pressure, or Pco2? [What does it mean that alkalinity is conservative?].
4) Is alkalinity conserved with boiling?
5) Can you raise the total alkalinity of a solution that has a fixed pH?
2) What is total alkalinity (ANC)?
3) Does alkalinity change with temperature, pressure, or Pco2? [What does it mean that alkalinity is conservative?].
4) Is alkalinity conserved with boiling?
5) Can you raise the total alkalinity of a solution that has a fixed pH?
In the strictest (correct) sense, “alkalinity” refers to total alkalinity (ANC, see number #2). In the normal pH range of shallow groundwater, as well as human blood, the predominant component of total alkalinity is bicarbonate. Unfortunately “alkalinity” has thus become synonymous with “bicarbonate,” which often leads to confusion in communication when discussing the conservation of alkalinity (see #3).
In the strictest (correct) sense, “alkaline” refers to a solution that has a positive total alkalinity. In pure water equilibrated to the atmosphere (i.e. precipitation) at standard temperature and pressure (STP), alkaline solutions range from pH 5.6 and higher. Unfortunately “alkaline” has incorrectly become a term referring to any pH greater than 7, where pH 7 is the neutral point of pure water without any dissolved carbon at STP. Unfortunately “alkaline” has also incorrectly become a term referring to the opposite of acidity, i.e. “basicity”. These uses should be avoided. Though it is a continuously increasing function of pH, TOTAL ALKALINITY IS NOT A LINEAR FUNCTION of pH AND DOES NOT START AT pH 7.
Total alkalinity quantifies the amount of acid that can be neutralized in solution, thus total alkalinity is also known as the acid neutralizing capacity (ANC). Total Alkalinity is defined as “the sum of bases in equivalents (or milli-equivalents) titratable with strong acid.” Total alkalinity can be expressed as an intensive property of a solution as equivalents per liter (or milli-equivalents per liter, mEq/L).
Total Alkalinity has several components, including excess hydroxide base expressed as (OH– – H+), the direct acid neutralizing capacity; and the buffers, bicarbonate (HCO3–) and carbonate (CO32-), the buffering capacity.
Total Alkalinity [ANC] = [HCO3-] + 2[CO3–] + [OH-] – [H+]
where brackets [ ] are concentrations. In some natural waters, other bases that act as buffers may be present in significant enough quantities that they must be included in the calculation of total alkalinity. These include dissolved silicates, borates, ammonia, organic bases, sulfides, and phosphates.
Inorganic carbon, initially in the form of dissolved CO2, forms the weak acid H2CO3 (carbonic acid), which gives rise by dissociation to its conjugate bases, bicarbonate and carbonate, that act as buffers. Unlike with the direct acid neutralizing capacity, the buffering components neutralize incoming (added) acid or base without changing the pH, although total alkalinity does change.
The video above shows the alkalinity components in water in equilibrium with a constant total carbon (Ct) concentration of 1 x 10-3 moles / liter, the same concentration as H+ at a pH of 3 and of OH– at a pH of 11, the endpoints of the graph. Throughout the pH domain of the graph, equilibrium concentrations of the different species of dissolved carbon [carbonic acid, bicarbonate, and carbonate] as well as H+ and OH– are plotted. The equilibrated level of the water with zero [negligible] alkalinity is where HCO3– = H+, at a pH of 4.7. For comparison, a water in equilibrium with 1981 levels of atmospheric CO2 (315 ppm) has a lower total dissolved carbon (Ct) concentration of approximately 1.2×10-5 moles/liter, with a resulting pH of 5.6 at the point of negligible alkalinity, the pH of pure water (with no other acid) precipitation. Most commonly, as bases weathered from rocks are added to the water in the soil, the pH of the water quickly rises above 7, and alkalinity increases with rising levels of first bicarbonate, and then carbonate, in equilibrium with the rising pH.
The video shows the changing levels of carbon species from an alkalimetric titration, the steady addition of milliliters (mL) of hydroxide base into a liter of water containing this constant amount of inorganic carbon (0.001 moles / liter). Carbonic acid, bicarbonate, and carbonate exchange places as their relative equilibrium levels change with the increasing pH.
Things to note in the video above:
a) Total alkalinity (in red) is a continuously increasing function of pH, BUT NOT A LINEAR FUNCTION of pH.
b) As the carbonate components exchange places, they neutralize base (in an alkalimetric titration … in an acidimetric titration they neutralize acid), thus “resist the pH change.” This is represented by steep ramps on the titration curve (in blue).
c) Once a buffering component is consumed, there is a rapid pH change until the next buffering component takes over. This is represented by gradual flats on the titration curve (in blue).
d) Without carbonate buffers, 1 mL of 1 molar OH- added to a liter of pure water would result in an OH- concentration of 0.001 corresponding to a pH of 11. By the end of the titration, pH 11 is only reached after about 3 mL of 1 molar OH- has been added. Thus the buffering capacity (as HCO3- + CO3–) of the total alkalinity is twice the direct neutralization capacity.
#3) Does alkalinity change with temperature, pressure, or Pco2? [What does it mean that alkalinity is conservative?].
No, total alkalinity [ANC] does NOT change with temperature, pressure, or Pco2. In other words, total alkalinity is conservative. But individual components of the sum may / will change, maintaining the sum, including bicarbonate, which confusingly is often called “alkalinity.”
Total Alkalinity [aka Acid Neutralizing Capacity, ANC], is defined as:
Total Alkalinity [ANC] = [HCO3-] + 2[CO3–] + [OH-] – [H+]
Where brackets [ ] are concentrations, and in words as, “the sum of bases, in equivalents (or milliequivalents), titratable with strong acid,”
ANC is a conservative property, which means it does not change with temperature, pressure, or Pco2. However, its individual components can change while maintaining the constant sum.
Total alkalinity can be changed under conditions of fixed pH by changing the amount of total inorganic carbon (abbreviated TIC or Ct) dissolved in solution.
Total alkalinity will only change with a chemical reaction involving an acid or base, or the addition of on acid or base to the solution.
Total alkalinity is conserved, even with boiling, so long as the solution is not excessively evaporated, but not necessarily bicarbonate. As you raise temperature, the equilibrium constants will change according to the Van’t Hoff equation, thus the balanced concentrations in solution will change, thus the pH will change, but the ANC (in milliequivalents) will not. It takes a chemical reaction with an acid or base, or an addition of an acid or base, to change ANC (total alkalinity).
Boiling will exsolve and liberate CO2 from solution, which will cause effective Pco2 to drop (if unconfined). This in turn will cause bicarbonate alkalinity, HCO3-, to drop, but as HCO3- drops by an “equivalent” amount, H+ (acid) will drop by the same “equivalent” amount and pH will rise. Thus TOTAL alkalinity (the sum) will be unchanged (conserved).
Yes, but it requires acid neutralization in the process. You can raise levels of total alkalinity even under conditions of fixed pH, including blood pH homeostasis.
Remember, total alkalinity is NOT “basicity,” i.e. it is NOT defined as a pH > 7 nor pOH. It is, in words, “the equivalent sum of bases titratable with strong acid, as measured in equivalents,” and it is a function of pH and Total Inorganic Carbon [abbreviated TIC or Ct]. It is also known as the acid neutralizing capacity (ANC), of which the carbonate components provide a buffering capacity.
As the graph indicates, a higher alkalinity at a given fixed pH has a higher TIC, but raising TIC alone does NOT increase alkalinity, and it drops pH.
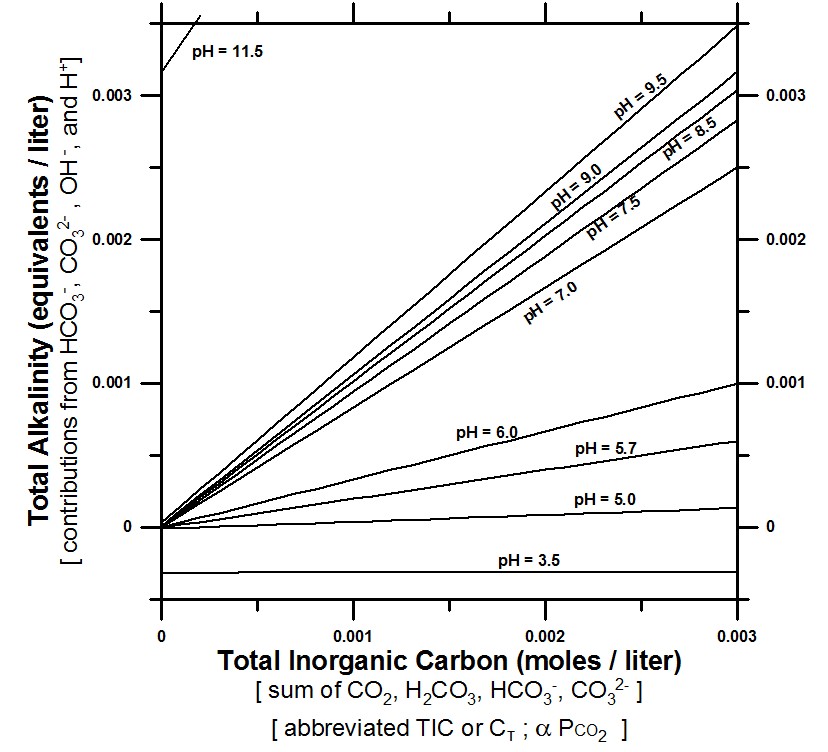
Two components must be changed together to raise total alkalinity at a given fixed pH: 1) TIC must be increased (the body does this by increasing dissolved CO2 with respiration), and 2) pH must be fixed by either simultaneously consuming acid (incorporation of acid into hemoglobin may do this), or adding base OH- (Kangen water has hydroxyide alkalinity OH-). Added base OH- converts increased CO2 (increased TIC) to HCO3- by this reaction: CO2 + OH- => HCO3- which both raises TIC and maintains the pH.
The graph is a partial reconstruction of the graph by geochemist Kenneth Deffeyes (1965), but using pH’s of interest to the electro-reduced water (ERW) community, including pH settings of Enagic’s SD 501 for making Kangen water. The Deffeyes reference is:
Kenneth S. Deffeyes; Carbonate equilibria: A graphic and algebraic approach. Limnology and Oceanography, 10 (1965), pp. 412–426.
pH values of interest on the graph include:
pH 2.5 Enagic strong acidic water (compliment to strong Kangen water); reported pH of Coca Cola due to presence of phosphoric acid (not due to carbonation alone).
pH 3.5 example acidic pH in range of graph
pH 5.0 acidic pH just below the CO2 equilibrated level
pH 5.7 pH of solution equilibrated to atmospheric CO2 (pH of rain)
pH 6.0 Enagic beauty water
pH 7.0 neutral water
pH 7.5 approximate upper limit of blood (pH = 7.45)
pH 8.1 pH of an equilibrated baking soda (NaHCO3) mixture (not plotted)
pH 8.2 pH of seawater (not plotted)
pH 8.5 Enagic Kangen drinking water 8.5
pH 9.0 Enagic Kangen drinking water 9.0
pH 9.5 Enagic Kangen drinking water 9.5
pH 11.5 Enagic strong Kangen water (compliment to strong acidic water)
pH 2.5 Enagic strong acidic water (compliment to strong Kangen water); reported pH of Coca Cola due to presence of phosphoric acid (not due to carbonation alone).
pH 3.5 example acidic pH in range of graph
pH 5.0 acidic pH just below the CO2 equilibrated level
pH 5.7 pH of solution equilibrated to atmospheric CO2 (pH of rain)
pH 6.0 Enagic beauty water
pH 7.0 neutral water
pH 7.5 approximate upper limit of blood (pH = 7.45)
pH 8.1 pH of an equilibrated baking soda (NaHCO3) mixture (not plotted)
pH 8.2 pH of seawater (not plotted)
pH 8.5 Enagic Kangen drinking water 8.5
pH 9.0 Enagic Kangen drinking water 9.0
pH 9.5 Enagic Kangen drinking water 9.5
pH 11.5 Enagic strong Kangen water (compliment to strong acidic water)
Note: The total alkalinity of “strong Kangen water” (pH 11.5) is almost entirely from OH- when TIC is 0. The other pH levels go to ~0 when TIC goes to zero and are thus manageable by the human body through respiration, for example pH = 9.5, the highest drinking setting of the SD 501
Note: TIC is proportional to Pco2. The graph assumes an open system where CO2 can be increased by dissolution of CO2 gas.
Note: Most additional total alkalinity is converted to bicarbonate alkalinity, HCO3-. In addition to blood pH homeostasis there is blood bicarbonate (HCO3-) homeostasis, so any boost to blood alkalinity will eventually be moderated to normal blood levels. For arterial blood gas, normal bicarbonate levels range from 22 to 26 mEq/L

